Legal & Safety Information (Accessories)
CE mark
Table of contents
LAST UPDATED: Jan 18, 2023
The Instructions for Use for Byteflies Kit Accessories, developed by Byteflies, are listed here. Byteflies Accessories were specifically designed for use with a Byteflies Kit but can also be used with certain 3rd party devices. Similarly, Byteflies Kit can also be used with certain compatible 3rd party accessories. Use with with 3rd party devices is not officially supported by Byteflies and it is up to the User to review all relevant manufacturer information.
The following official Byteflies Kit Accessories are available:
- ECG Adhesive
- A single-use disposable skin adhesive with embedded electrodes, meant to record ECG channels when used in combination with a compatible biopotential recording device, such as Byteflies Kit.
- EEG Adhesive
- A single-use disposable skin adhesive with two embedded electrodes, meant to record a single EEG channel when used in combination with a compatible biopotential recording device, such as Byteflies Kit.
- CardioCare@Home Pro
- A software accessory for healthcare professionals that can visualize data recorded by a compatible medical device, such as Byteflies Kit.
- EpiCare@Home UI
- A software accessory for healthcare professionals that can visualize data recorded by a compatible medical device, such as Byteflies Kit.
ECG Adhesive
Intended Use
The Byteflies ECG Adhesive is a single-use biopotential electrode device intended to record electrocardiogram (ECG) signals. It can be used in healthcare, home, and research environments. The ECG Adhesive is intended to be used on intact (uninjured) skin and can be worn for up to 3 days.
Intended Users
ECG Adhesives are intended to be used by patients 3 years of age and older under the instruction of a trained operator. ECG Adhesives are single-use and can be applied at home by a user. Any recording equipment connected to the ECG Adhesive should be configured by a trained operator according to its instructions for use.
Clinical Indications
ECG Adhesives are intended for use in the home, healthcare facility, or clinical research environment to acquire ECG signals from the user. ECG Adhesives are compatible with biopotential recording devices that can connect to 4 mm snap fasteners.
Safety Information
The following is important information for using ECG Adhesives properly and safely. Carefully read this section before using or instructing to use ECG Adhesives. ECG Adhesives are meant to be used with a compatible biopotential recording device, such as a Byteflies Kit. Refer to the relevant manufacturer instructions to verify it is compatible with ECG Adhesives.
- Observe recommended procedures of electrosurgical or monitoring equipment manufacturers to minimize risk of burns at ECG Adhesive sites during electrosurgical procedures.
- Do not place the ECG Adhesive on skin with established erythema, lesions and injuries of any kind.
- Do not use on users with visible skin irritation in direct ECG Adhesive contact and report any observed incompatibility reactions to Byteflies.
- Do not use an ECG Adhesive for more than 72 hours.
- The ECG Adhesive is for single use only. If re-used, the adhesive and the electrical properties might be insufficient.
Warnings

Do not use ECG Adhesives before reading these warnings:
- One or more ECG Adhesives are provided in a non-sterile pouch, as indicated on the product label.
- Do not use an ECG Adhesive if the adhesive itself or its pouch is damaged, or if the shelf-life is expired, as indicated on the product label.
- Keep any unused ECG Adhesives in the original pouch. Close the opened pouch and fold the top of the pouch twice. Do not use ECG Adhesives if the pouch was opened more than a month ago.
- Place ECG Adhesives as instructed by a trained operator specialized in ECG monitoring and diagnosis.
- Ensure that the user’s skin is dry, clean, and free of excessive hair. Do not use solvents or abrasive materials to clean the skin prior to application of the ECG Adhesive as that may lead to skin irritation.
- Connect a compatible biopotential recording device, such as a Byteflies Kit, to the 4 mm snap fasteners to record the ECG channels. Refer to the manufacturer’s instructions to ensure the ECG Adhesive is compatible with the recording device.
- If the user experiences any adverse events while using an ECG Adhesive, discontinue its use and contact Byteflies immediately.
- An ECG Adhesive should be replaced if they no longer stick firmly to the skin or within 72 hours of use, whichever happens sooner.
- Take extra care when removing an ECG Adhesive from overly delicate skin (e.g. in the case of elderly patients or due to prolonged use of medications like steroids) to avoid skin damage.
- ECG Adhesives are single-use, do not attempt to re-use or clean.
- Do not use at temperatures lower than 10°C or greater than 40°C.
- Do not damage any component of an ECG Adhesive.
- ECG Adhesives are not toys. Usage on children 3 years of age and older should be under strict supervision of an adult.
- Do not alter any component of an ECG Adhesive. Any modification is strictly prohibited.
Package and Device Symbols
| Symbol | Description |
|---|---|
 | Manufacturer |
 | Date of manufacture |
 | Catalog number |
 | LOT number |
 | Medical device |
 | Unique device identifier |
 | CE marking of conformity |
 | Warning |
 | User should consult the instructions for use. |
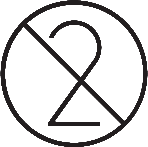 | Do no re-use. |
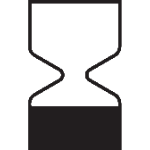 | Do no use past the shelf-life date. |
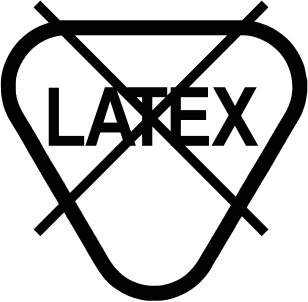 | Device is not made with natural rubber latex. |
 | Keep the device dry; the presence of liquids may compromise the safety of the device. |
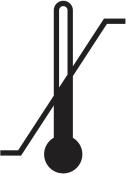 | The maximum and minimum temperature limits to which the device or accessory should be exposed during use. |
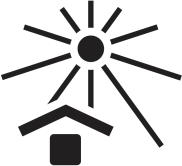 | Keep out of direct sunlight. |
EEG Adhesive
Intended Use
The Byteflies EEG Adhesive is a single-use biopotential electrode device intended to record electroencephalogram (EEG) signals behind the ears. It can be used in healthcare, home, and research environments. The EEG Adhesive is intended to be used on intact (uninjured) skin and can be worn for up to 2 days.
Intended Users
EEG Adhesives are intended to be used by patients 3 years of age and older under the instruction of a trained operator. EEG Adhesives are single-use and can be applied at home by a user. Any recording equipment connected to the EEG Adhesive should be configured by a trained operator according to its instructions for use.
Clinical Indications
EEG Adhesives are intended for use in the home, healthcare facility, or clinical research environment to acquire EEG signals from the user. EEG Adhesives are compatible with biopotential recording devices that can connect to 4 mm snap fasteners.
Safety Information
The following is important information for using EEG Adhesives properly and safely. Carefully read this section before using or instructing to use EEG Adhesives. EEG Adhesives are meant to be used with a compatible biopotential recording device, such as a Byteflies Kit. Refer to the relevant manufacturer instructions to verify it is compatible with EEG Adhesives.
- Observe recommended procedures of electrosurgical or monitoring equipment manufacturers to minimize risk of burns at EEG Adhesive sites during electrosurgical procedures.
- Do not place the EEG Adhesive on skin with established erythema, lesions and injuries of any kind.
- Do not use on users with visible skin irritation in direct EEG Adhesive contact and report any observed incompatibility reactions to Byteflies.
- Do not use an EEG Adhesive for more than 48 hours.
- The EEG Adhesive is for single use only. If re-used, the adhesive and the electrical properties might be insufficient.
Warnings

Do not use EEG Adhesives before reading these warnings:
- One ore more EEG Adhesives are provided in a non-sterile pouch, as indicated on the product label.
- Do not use an EEG Adhesive if the adhesive itself or its pouch is damaged, or if the shelf-life is expired, as indicated on the product label.
- Keep any unused EEG Adhesives in the original pouch. Close the opened pouch and fold the top of the pouch twice. Do not use EEG Adhesives if the pouch was opened more than a month ago.
- Place EEG Adhesives as instructed by a trained operator specialized in EEG monitoring and diagnosis.
- Ensure that the user’s skin is dry and clean. Do not use solvents or abrasive materials to clean the skin prior to application of the EEG Adhesive as that may lead to skin irritation.
- Connect a compatible biopotential recording device, such as a Byteflies Kit, to the 4 mm snap fasteners to record one channel of EEG per EEG Adhesive used. Refer to the manufacturer’s instructions to ensure the ECG Adhesive is compatible with the recording device.
- If the user experiences any adverse events while using an EEG Adhesive, discontinue the use and contact Byteflies immediately.
- An EEG Adhesive should be replaced if they no longer stick firmly to the skin or within 48 hours of use, whichever happens sooner.
- Take extra care when removing an EEG Adhesive from overly delicate skin (e.g. in the case of elderly patients or due to prolonged use of medications like steroids) to avoid skin damage.
- EEG Adhesives are single-use, do not attempt to re-use or clean.
- Do not use at temperatures lower than 10°C or greater than 40°C.
- Do not damage any component of an EEG Adhesive.
- EEG Adhesives are not toys. Usage on children 3 years of age and older should be under strict supervision of an adult.
- Do not alter any component of an EEG Adhesive. Any modification is strictly prohibited.
Package and Device Symbols
| Symbol | Description |
|---|---|
 | Manufacturer |
 | Date of manufacture |
 | Catalog number |
 | LOT number |
 | Medical device |
 | Unique device identifier |
 | CE marking of conformity |
 | Warning |
 | User should consult the instructions for use. |
 | Do no re-use. |
 | Do no use past the shelf-life date. |
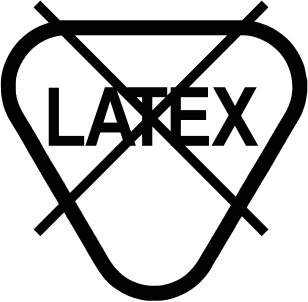 | Device is not made with natural rubber latex. |
 | Keep the device dry; the presence of liquids may compromise the safety of the device. |
 | The maximum and minimum temperature limits to which the device or accessory should be exposed during use. |
 | Keep out of direct sunlight. |
Regulatory Information
Declaration of Conformity
Byteflies ECG Adhesives are in conformity with the general safety and performance requirements of the EU Medical Device Regulation (MDR) 2017/745.
Byteflies EEG Adhesives are in conformity with the general saftey and performance requirements of the EU Medical Device Regulation (MDR) 2017/745.
Byteflies ECG and EEG Adhesive Compliant Regulatory Standards
- C
- The Byteflies ECG and EEG Adhesive is compliant with this standard.
- R
- The Byteflies ECG and EEG Adhesive reference this standard ("in the spirit of").
| Standard | Implementation | Title | Remarks |
|---|---|---|---|
| 2017/745 EU MDR (2017) | C | EU Medical Device Regulation | |
| EN ISO 13485:2016 + A11:2021 | C | Medical Devices - Quality Management Systems - Requirements For Regulatory Purposes | |
| FDA 21 CFR, part 820 | C | Quality system regulation for medical device, federal register | |
| EN ISO 14971:2019 + A11:2021 | C | Application of risk management to medical devices | |
| IEC 62366-1:2015 + AMD1:2020 | C | Medical devices - Part 1: Application of usability engineering to medical devices | |
| EN 62304:2006 + AMD1:2015 | C | Medical device software - Software life cycle processes | |
| ISO 20417:2021 | C | Medical devices - Information supplied by the manufacturer of medical devices | |
| EN ISO 15223-1:2021 | C | Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements | |
| EN ISO 14155:2011 | C | Clinical investigation of medical devices for human subjects – Good clinical practice | |
| ISO 10993-1:2018 | C | Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process | |
| ISO 10993-5:2009 | C | Biological evaluation of medical devices - Part 5: Test for in vitro cytotoxicity | |
| ISO 10993-10:2010 | C | Biological evaluation of medical devices - Part 10: Tests for irritation and skin sensitization | |
| ANSI/AAMI EC12:2000/(R)2020 | C(*) | Disposable ECG Electrodes | |
| MDCG 2020-5 rev 1 (April 2020) | C | Clinical Evaluation: Equivalence A guide for manufacturers and notified bodies | |
| MDCG 2019-15/rev.1 | C | Guidance notes for manufacturers of class I medical devices System | |
| MDCG 2020-1 | C | Guidance on clinical evaluation (MDR) / Performance evaluation (IVDR) of medical device software | |
| MDCG 2020-7 | C | Post-market clinical follow-up (PMCF) Plan Template | |
| MDCG 2020-8 | C | Post-market clinical follow-up (PMCF) Evaluation Report Template | |
| MDCG 2020-5 | C | Clinical Evaluation - Equivalence | |
| ASTM D4332:2014 | C | Standard Practice for Conditioning Containers, Packages or Packaging Components for Testing | |
| RoHS recast Directive 2011/65/EU (2011) + Amd Directive 2017/2102/EU | C | Restricting the use of hazardous substances in electrical and electronic equipment | |
| WEEE Directive 2012/19/EU | C | Waste Electrical & Electronic Equipment (WEEE) Directive | |
| FDA Guidance (2016) | R | Applying Human Factors and Usability Engineering to Medical Devices | |
| FDA Guidance (2016) | R | Use of International Standard ISO 10993-1, “Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process” | |
| FDA Guidance (2019) | R | Safety and Performance Based Pathway | |
| FDA Guidance (2016) | R | Unique Device Identification System: Form and Content of the Unique Device Identifier (UDI) | |
| NBOG BPG 2014-3 (2014) | R | Guidance for manufacturers and Notified Bodies on reporting of Design Changes and Changes of the Quality System |
(*) Byteflies EEG Adhesive is partially compliant with this standard